Process analytical technology
Process Analytical Technology, abbreviated PAT, is a framework presented by the American Food and Drug Administration in 2004. The intention of the framework is to encourage supervising the design, analysis, and quality control of pharmaceuticals and food to ensure final product quality.
The PAT framework operates by monitoring and adjusting the Critical Process Parameters (CCP) in order to identify and control the Critical Quality Attributes (CQA). By defining the CPPs and accordingly monitoring them in a timely manner e.g. in-line, one begins to understand the process and will be more sufficient in testing while at the same time reducing over-processing, enhancing consistency, and minimizing rejects.
In order for the PAT to contribute to maintaining good quality control, you need high-quality data. These data can be obtained by a near-infrared (NIR) spectrometer.
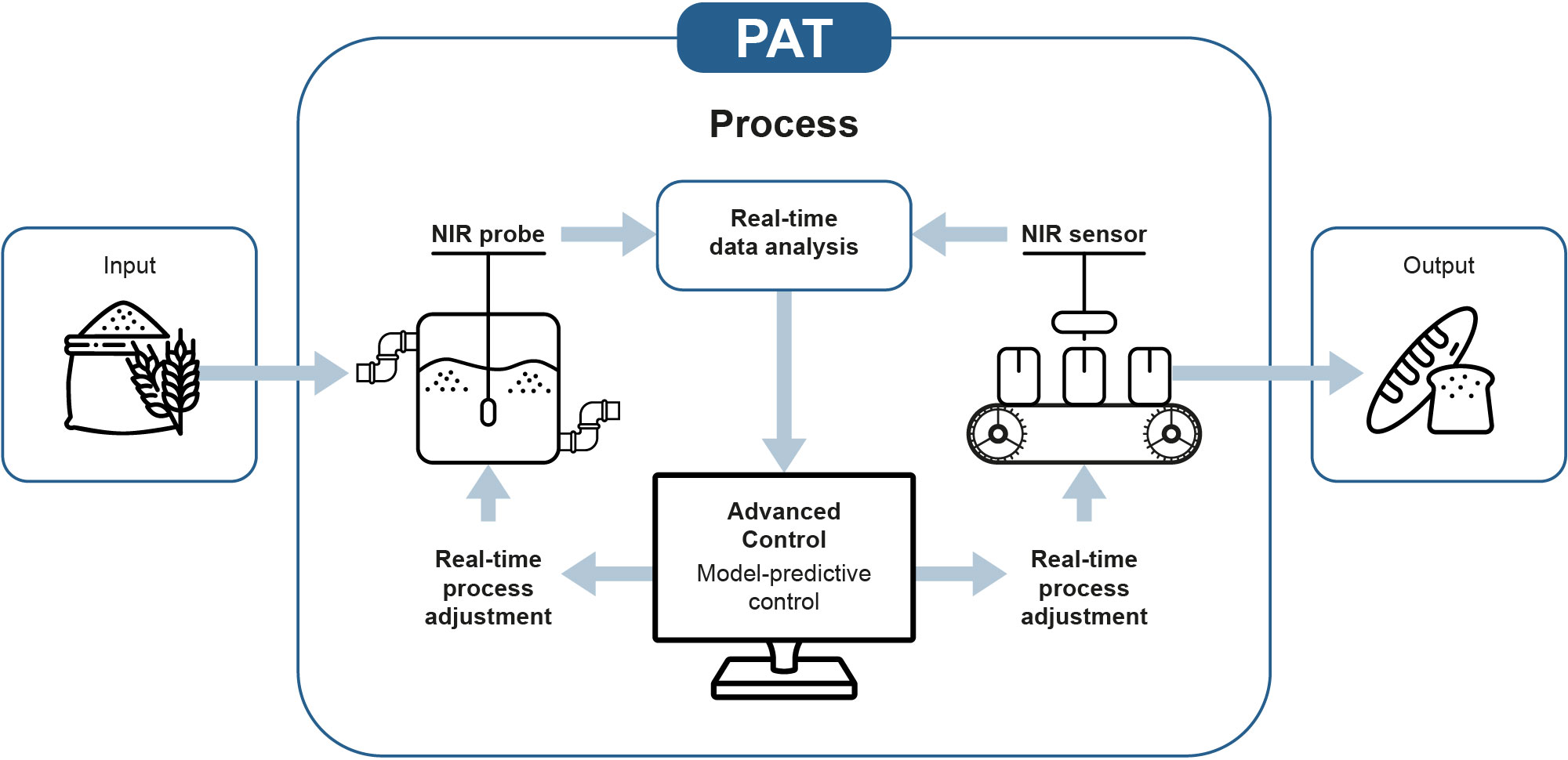
One of the benefits of in-line control versus off-line is the immediate process feedback and ability control and adjusts the production instantaneously rather that after the delay off-line processes often causes. This ensures an improved product quality control which is merely the purpose of PAT.
The recommended analytic method used for PAT is NIR spectroscopy. The infra-red range is preferred because many of the organic molecules used in food and pharma have absorption in this range. NIR spectrometers, like Ibsen’s ROCK NIR, and ROCK XNIR, enable fast, real-time, and non-destructive measurements of samples. Furthermore, such spectrometers are very stable against vibration and thermal changes and are therefore very well suited for use directly on the factory floor.
Do you want to know more?
Want to know more?
For further information see below.